Combisal® is a pressurised metered-dose inhaler (pMDI), indicated in the regular treatment of asthma where use of a combination product (long-acting β2 agonist and inhaled corticosteroid) is appropriate:
- patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short- acting β2 agonist
or
- patients already adequately controlled on both inhaled corticosteroid and long-acting β2 agonist1
Combisal® – flexibility and consistency for patients and prescribers, every step of the way.
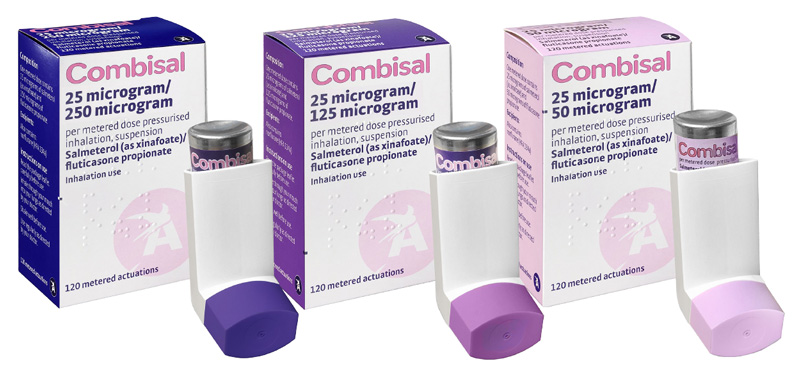
All equivalent strengths to Seretide Evohaler® (salmeterol/fluticasone)
- Combisal is available in the same three strengths and indications as the brand originator Seretide Evohaler® pMDI, providing significant opportunities for retail pharmacy and potential cost savings for the NHS.
- Combisal® 25µg/50µg pressured metered dose inhaler
- Combisal® 25µg/125µg pressured metered dose inhaler
- Combisal® 25µg/250µg pressured metered dose inhaler
- Combisal provides the ability to cost-effectively manage asthma within the BTS guidelines4 by stepping up and down the steroid dose, without the need to change brands
- The ability to step up and down within the range aids compliance with dosing for patients and prescribers alike.
Licensed for Paediatrics and Adolescents°
- Combisal is equivalent to Seretide® pMDI (salmeterol/ fluticasone) and can be prescribed in children aged 4 years and over (Combisal® 25/50μg)1-3*†
- Combisal is equivalent to Seretide® pMDI (salmeterol/ fluticasone) and can be prescribed in patients under 18 years of age. Available to prescribe in patients from 12 years (Combisal® 25/125µg, 25/250 µg), Combisal provides the ability to cost effectively treat under 18s that require higher doses of steroid1,2

*The maximum licensed dose of fluticasone propionate delivered by Combisal inhaler in children is 100µg twice daily. Combisal 25/50μg is not appropriate for adults and children with severe asthma. #Contains alcohol. † Use of an AeroChamber Plus spacer device with Combisal is recommended in those who may have difficulties coordinating actuation with inspiration e.g. children <12 years. °Children aged 4 years and over can be prescribed Combisal® 25/50μg, with adolescent patients from 12 years prescribed Combisal® 25/125μg and 25/250 μg strengths.
References: 1) Combisal Summaries of Product Characteristics. 2) Seretide Evohaler Summaries of Product Characteristics. 3) Bioequivalence Data on File. 1010422379 v 3.0 April 2021. 4) British Guideline on the Management of Asthma. British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. SIGN 158. BTS/SIGN, 2019. Available at: https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma. (Accessed January 2023)
COM1010422E1_JAN2023


















